Our pipeline of SPEARs™ target transcription factors and regulatory pathways of disease that make cells cancerous or dampen the immune system against tumors
Pipeline
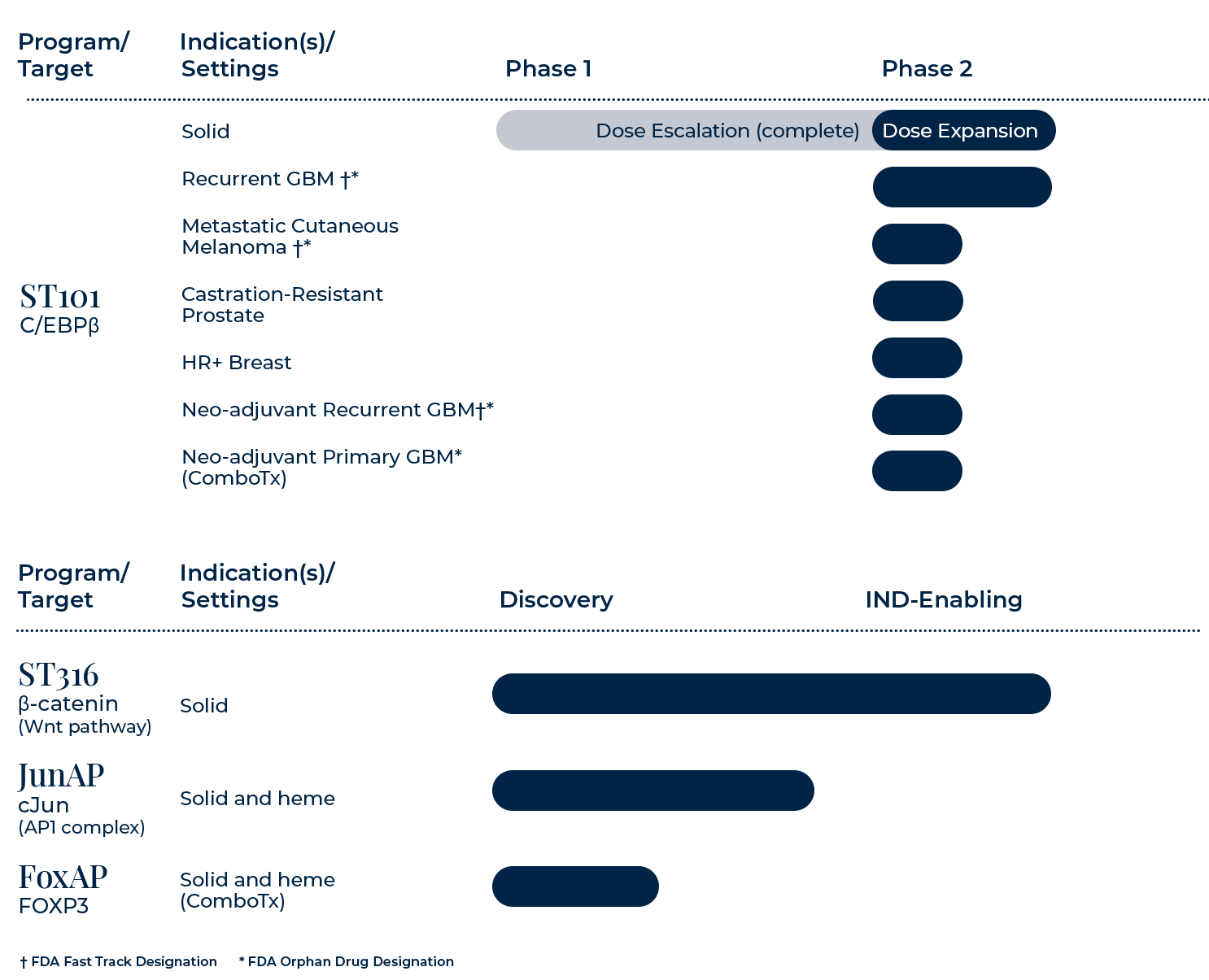
ST101
ST101 is a first-in-class antagonist of C/EBPβ, a development stage transcription factor that turns oncogenic and immunosuppressive when active post-differentiation. ST101 is currently being evaluated in the Phase 2 portion of an ongoing Phase 1-2 study in patients with advanced unresectable and metastatic solid tumors.
View ST101ST316
ST316 is a first-in-class antagonist of β-catenin currently in IND-enabling studies. β-catenin is a critical member of the canonical WNT signaling pathway, a well-known development stage pathway.
View ST316cJun Program
cJun and cFos form one of the most formidable, yet desired, undruggable targets, the AP-1 complex. Data demonstrates that our cJun SPEAR candidates cause in vitro cell killing and in vivo anti-cancer activity.
View cJun ProgramFoxP3 Program
FoxP3 functions as a master regulator for the development and function of regulatory T cells (Tregs), a subpopulation of T cells that modulate immune responses by suppressing or downregulating induction and proliferation of effector T cells. Tregs contribute to cancer by suppressing T effector cells, thereby compromising tumor killing and promoting tumor growth. Data suggests that our FoxP3 peptide hits have potent inhibition of FoxP3 gene transcription by in vitro reporter assay.
View FoxP3 ProgramExpanded Access
at BioAce Health we are committed to developing safe and effective therapies for high mortality cancers and making them available to patients as quickly and efficiently as possible by conducting rigorous clinical trials needed to gain regulatory approval. We support expanded access to our investigational medicinal products when there is substantial scientific evidence to assure both safety and efficacy. Early in development the risks of expanded access tend to outweigh the benefits due to limited data. Thus, evaluation of requests will generally require availability of positive Phase 3 data as well as meeting program and patient eligibility criteria.
View Expanded Access